Erodium cicutarium - a plant catapult and moving seeds
The following article was originally published in Biologia w Szkole (5/2020), a journal for teachers and lecturers: (5/2020):

In this article, I would like to keep on with the topic of movement in the plant kingdom. In our earlier research, we have already learned that plants are capable of moving some parts of their bodies with respect to the others. We also know that the causes and goals of these movements, as well as their mechanisms, are completely different than those employed by animals. Such differences are due to the different physiology of the Animalia and Plantae kingdoms resulting from different evolutionary pathways.
The major difference between plants and animals is easy to spot at first glance: plants are not capable of locomotion (i.e. the autonomous movement of the whole organism). If plants were not able to move from one place to another like animals, then how would they conquer new areas?
The spread of diasporas is the plants' most important mechanism of colonizing new habitats, while the movement of individuals remains almost negligible. This term (greek: dia - through, sporos - seed) refers to any plant part that is used for reproduction and spreading. In a similar sense diasporas are also produced by fungi [1]. Therefore, a diaspora can both present the whole organism, and the organism’s part capable of producing a new individual. Diasporas are divided into generative (seeds) and vegetative (fragments of thallus, tubers, rhizomes, turions, seedlings, spores) [2].
Plants are capable of many different types of motion. Examples include catching small animals as food (venus flytrap Dionea muscipula, cape sundew Drosera capensis), for pollination (european barberry Berberis vulgaris) and protecting flowers against environmental conditions (strawflower Xerochrysum bracteatum). These movements are, generally, quite slow [3][4][5]. In order to observe the phenomenon of plant movement, we usually have to use the technique of time-lapse photography.
As it turns out, there are plant organs of which have the ability to move at speeds so high that use of special filming techniques is necessary to slow down the recorded video and enable observation. The seeds of the plant I want to tell about have the ability to independently… bury themselves in the ground. Interestingly, the mentioned organism does not belong to any exotic species and can be found in our country, Poland.

Erodium
Erodium cicutarium was the first species belonging to the Geraniaceae family I scrutinized in my laboratory. Depending on the source, the genus Erodium includes from 60 to as many as 130 confirmed species [6][7]. Erodium sp. usually live in not very hospitable places, i.e. sandy and rocky terrain. Southern Europe has the greatest species diversity when it comes to those plants, but they can also be found on other continents. Erodium cicutarium, which we are interested in, is found in the wild in Poland (most likely as an archaeophyte), but some related species are sometimes cultivated as ornamental plants. Besides that, it is spread everywhere except Antarctica.
For some reasons, which will become clear in a moment, Erodium is commonly referred to as stork’s nose - other names related to a bird's beak are also often used.
Erodium cicutarium clearly prefers acidic and light, sandy or sandy-loam soils, and at the same time rich in nutrients; it is definitely a nitrogen-loving plant. It can be found in areas used for agriculture, which is why it is considered a weed in cultivation of root crops and cereals, including maize. It can also be seen in clover Trifolium fields. The presence of Erodium in crops may lead to excessive drying of the soil and its nutrients depletion. Additionally, its rapid expansion often causes slowdown in growth or even death of seedlings of other plants. Thanks to its adaptations Erodium can also reproduce very efficiently and spread its seeds over a relatively large area [8][9].
In our climate (Poland), Erodium is a relatively small annual plant. I admit that despite its wide distribution in nature, I had to spend some time finding specimens for observation. Interestingly, I discovered them in my garden, where plants were apparently sown by accident (Photo.1).
Erodium cicutarium is not a protected by law, so for more precise observation we can take it from the environment in parts or as whole (Photo 2). Of course, a specimen of this interesting plant can also be a decoration for our herbarium.
Stem of this plant is usually erect and covered with long, soft hairs, and in the upper part also with glands. Leaves growing close to the soil form a rosette, while the stem leaves grow alternately and are getting smaller upwards (Photo 3).
Small flowers on long stalks are gathered three to ten in umbel-like inflorescences. They are delicately zygomorphic, purple, sometimes white or with lighter spots, with five egg-shaped petals, clearly longer than the sepals. There are five stamens and the same number of staminodes (Photo 4).
The sepals are glandularly hairy, lanceolate or oblong, with membranous edges (Photo 5).
Erodium cicutarium, like other Geraniaceae, is pollinated by insects, that is its flowers have small nectaries. After pollination, a fruit is formed and it shows interesting structural features and surprising behavior.
Natural machine
The fruits of Erodium cicutarium (also of other species of this genus) are very characteristic. Their shape can indeed bring to mind a needle or a stork's beak, which gave rise to both botanical and common names of this plant (Photo 6).
Said fruit should be classified as schizocarp with elongated tail-shaped structure (Photo 7). It is easy to see the remains of sepals, as well as mature mericarps.
Mature and dry schizocarp easily breaks down into 5 mericarps separating from the central elongated tail-shaped element (Photo 8). Each of them has a long, thin awn.
Inside each mericarp, one brown smooth surfaced seed can be found (Photo 9). The plant produces several hundreds of them, usually 200-600.
The way plants of the genus Erodium have adapted to the living conditions and developed suitable dispersal methods is striking.
We know that plants show variety of seed dispersal strategies. First, we can mention an allochory characterized by use of various external factors for spreading their seeds. Within this category, we can distinguish:
- hydrochory; by water (especially flowing),
- anemochory; by wind,
- zoochory; with the help of animals, for example ornitochory (via birds), myrmekochory (via ants) and others.
- antropochory; due to human activity.
Nevertheless, there is a set of mechanisms which do not involve external factors in spreading diasporas. It is called autochory (self-seeding), and it can be divided into:
- blastochory; by growing shoot lengthwise and leaving seeds some distance from the mother plant,
- barochory; gravitational, by direct drop of seeds to the ground,
- ballochory; by means of explosive mechanisms,
- herpochory; as a result of performing independent movements [10].
Interestingly, plants of the genus Erodium - and thus also Erodium cicutarium - use two types of autochory simultaneously, namely ballochory and herpochory… Yes! This plant can catapult its seeds (or rather mericarps) into the air by itself, and they themselves have the ability to move autonomously. So let's take a closer look at them (Photo 10).
As we can see, the two mericarps presented above differ in the shape of their awns. This is related to the level of water saturation: the hydrated awn (placed in humid air condition) is relatively straight, but as it dries, it bends significantly. This process is reversible and it is possible to repeat the wet-dry cycle (straight-bent awn) multiple times. The plant uses this movement to spread its seeds.
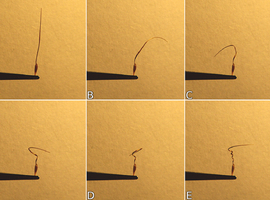
Before the schizoscarp breaks down, mericarps form a structural unity with the other tissues. In the mature fruit, the connection of mericarps with the rest of the fruit is quite delicate and acts as a trigger. Was the mericarp not immobilized, its awn would have simply bent as it dried. However, in the described case, it is impossible because of the connection of the awns along their entire length with the remaining tissues of the schizocarp - stress is generated in structures responsible for the flexion movement. Because of that, the plant accumulates potential energy that can be released by even a gentle touch of a ripe and dry fruit or by movements caused by the wind. Amount of stored energy is so great that said movement can happen even spontaneously, when the threshold stress value is exceeded. This phenomenon is so fast that we cannot see any details of it with the naked eye. Fortunately, having access to a high-speed digital camera, I was able to record this (Photo 11). I would like to point out that I used almost four thousand frames per second (4000fps), while under normal conditions recording rate is usually twenty-five to sixty frames per second (25-60fps). I conducted the experiment by placing the mature fruit of Erodium cicutarium in a holder made of metal tweezers attached to a stand and then gently touching with a needle. If the fruit is dry enough, even a slight contact will initiate a reaction.
As we can find out, the result of a delicate mechanical stimulus was literal ejection of the seed due to the stresses accumulated in the awn, which in consequence took an arcuate shape.
In the case of my experiments, the initial ejection speed of seed measured at a distance of first 10cm was about 3m/s (±1m/s, the high value of the standard deviation is most likely due to differences in humidity), which seems to be quite a good result, as for such a small plant. The available literature shows that the initial velocity may even exceed 4m/s, and the seeds are spread within a radius of 0.5m from the mother plant - which was also confirmed by my observations [11]. It is worth emphasizing once again that a single plant is able to densely cover an area of 1m in diameter around itself with its seeds. This fact alone can justify a considerable evolutionary success of Erodium sp., but this is not the only interesting adaptation. As I mentioned earlier, they use not only ballochory, but also herpochory.
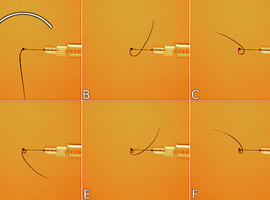
Not only does the awn allow ejection, but is also capable of multiple twists as it dries out. To perform the observations, immobilize the moistened (e.g. by storing in a chamber with a watered paper towel placed at the bottom) mericarp so that its awn remains free to move. During drying, we can notice quite quickly - because in just few minutes - twisting of the awn (Photo 12). The number of turns can reach up to nine.

The details of this movement are even easier to observe from the top (Photo 13). In this case, for the sake of clarity, the mericarp was immobilized by inserting an injection needle attached to a syringe placed on a stand.
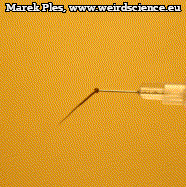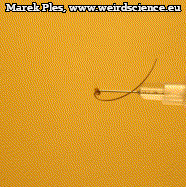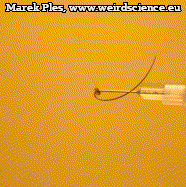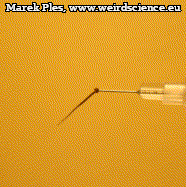
Twisting of the awn is always done in a way that makes the tip turn counterclockwise it is particularly easy to trace it in the montage made from photographs of subsequent movement stages (Photo 14).
The described mechanism of hygroscopic movements allows Erodium seeds to bury themselves in the ground. As the humidity changes (e.g. day-night cycle), the awn of mericarp straightens and twists alternately. Its free, non-bending tip can be fixed, for example, by catching it with the shoots of neighbouring plants. In such a case, the opposite, sharpened end of mericarp containing the seed is set into rotation, and thus screwed into the soil. Thanks to the successive cycles of changes in humidity, the seed penetrates into the soil to a depth of several millimeters, which protects it sufficiently, and provides favourable conditions for survival and germination.
Explanation
The shape changes of the awn result from hygroscopic movements. Structures showing such properties are usually built as double-layered systems. These layers react to presence of water with an unequal change in volume, which causes the entire structure to bend. Described structure is characteristics and occurs also in other plants. An example may be scales of female spruce cones Picea, as well as the elaters of horsetail spores Equisetum arvense [12]
Mainly specialized dead tissues take part in hygroscopic movements - therefore they do not require energy and are supplied from the external environment through changes in humidity. However, it must be kept in mind that creation of such complex structures is associated with large energy expenditure. The possibility of spreading over a relatively large area allowed by ballochory or providing the seeds with safe and favorable conditions through herpochory apparently gives the plant such great advantages that this evolutionary solution made Erodium successful.
Literature:
- [1] Szweykowska A., Szweykowski J., Słownik botaniczny (wyd. II, zmienione i uzupełnione), Wiedza Powszechna, Warszawa, 2003, str. 241
- [2] Kuta E., diaspora, w: Otałęga Z., Encyklopedia biologiczna, Agencja Publicystyczno-Wydawnicza Opres, Kraków, 1998
- [3] Ples M., A jednak się porusza! Ruchy higroskopowe roślin, Biologia w Szkole, 3 (2016), Forum Media Polska Sp. z o.o., str. 52-56
- [4] Ples M., O rosiczce słów kilka, czyli wyhoduj żywą muchołapkę!, Biologia w Szkole, 1 (2016), Forum Media Polska Sp. z o.o., str. 51-56
- [5] Ples M., Roślinny bokser? Szybkie ruchy pręcików berberysu, Biologia w Szkole, 3 (2020), Forum Media Polska Sp. z o.o., str. 81-85
- [6] Philips R., Rix M., The Botanical Garden (vol. 2), Macmillan, Londyn, 2011, str. 115
- [7] Erodium, w serwsie: http://www.theplantlist.org/, dostępne online: http://www.theplantlist.org/1.1/browse/A/Geraniaceae/Erodium/ [dostęp: 10.08.2020]
- [8] Paradowski A., Atlas chwastów, Plantpress, Kraków, 2013
- [9] Tymrakiewicz W., Atlas chwastów, Państwowe Wydawnictwa Rolnicze i Leśne, Warszawa, 1976
- [10] Vittoz P., Engler R., Seed dispersal distances: a typology based on dispersal modes and plant traits, Botanica Helvetica, 117 (2), 2008, str. 109-124
- [11] Evangelista D., Hotton S., Dumais J., The mechanics of explosive dispersal and self-burial in the seeds of the filaree, Erodium cicutarium (Geraniaceae), Journal of Experimental Biology, 214 (4), 2011, str. 521-529
- [12] Ples M., Skrzyp - roślina z przeszłości, Biologia w Szkole, 4 (2016), Forum Media Polska Sp. z o.o., str. 56-60
The author of the text and photos is Marek Ples.
Marek Ples