How to Make Water Boil at Room Temperature
| Polish version is here |
What Is Boiling?
In physics, boiling is the process of a liquid transforming into a gas. During boiling, bubbles of saturated vapor form within the liquid. While evaporation occurs at any temperature, boiling is unique because it occurs throughout the entire liquid, but only at its boiling point. Boiling requires a continuous input of energy, making it a first-order phase transition.
It’s important to note that boiling can occur at any temperature, as long as the pressure conditions are right. This is possible between the triple point and the critical point of a substance. For a given external pressure, a liquid boils at a specific temperature, known as its boiling point.
As external pressure increases, so does the boiling temperature, because the vapor pressure must equal the external pressure for boiling to occur. This relationship is described by the ideal gas law. Under standard atmospheric pressure (1 atm or 101.3 kPa), water boils at 100°C (212°F).
The boiling point is highly dependent on pressure. When the pressure is lower than 1 atm, water boils at a temperature below 100°C (212°F). This principle allows us to perform a simple but fascinating experiment!
Let’s Try It!
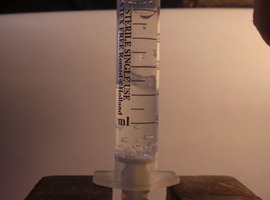
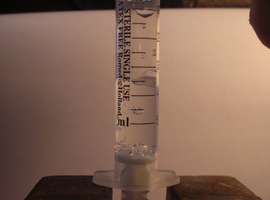
All you need is a new, airtight medical syringe. You can buy one at any pharmacy for just a few cents. A 5 mL syringe works best, but other sizes will work too.
The experiment is very simple to perform. First, draw a small amount of room-temperature water into the syringe—1 to 2 mL (0.03–0.07 fl oz) is enough. Press the plunger to remove any visible air bubbles from the syringe. Next, firmly seal the nozzle with your finger and pull the plunger back as hard as you can. If the syringe is airtight, the internal pressure will drop enough for the water to start boiling at room temperature, visible as the formation of vapor bubbles throughout the liquid.
Using the appropriate form of the ideal gas law, you can even calculate how much the internal pressure has decreased based on the water temperature.
Enjoy your experiment!
Further readings:
- Atkins P.W., Chemia fizyczna, Wydawnictwo Naukowe PWN, Warszawa, 2001
- Basiński A., Bielański A., Gumiński K., Chemia fizyczna. Wyd. 3, Państwowe Wydawnictwo Naukowe, Warszawa, 1966, pp. 297
- West J. B., Barometric pressures on Mt. Everest: New data and physiological significance, Journal of Applied Physiology, 1999, 86(3), pp. 1062–1066
Marek Ples