Peroxidase - The Enzyme Hidden Below Ground
| Polish version is here |
The following article was originally published in the journal for educators Biologia w Szkole (eng. Biology in School) (2/2019):

Enzymes, historically known as ferments, are one of the fundamental elements of this fascinating and essential set of physicochemical processes that constitute the material foundation of what we call life—not just for biologists, but for all of science. Their role is invaluable because without these substances, it would be virtually impossible for organisms to carry out metabolic reactions, and thus the transformation of matter and energy.
The field of biochemistry that focuses on enzymes is enzymology. It studies the structure of these substances, their properties, mechanisms of action, functions, biosynthesis, as well as methods for their extraction and purification. This is particularly important in medicine, as disturbances in enzyme activity are often the cause of diseases.
The specificity of enzymatic catalytic action is highly varied. Some enzymes act only on a specific substrate (for example, glucose oxidase oxidizes almost exclusively D-glucose), while others can act on a whole group of similar chemical substances. Peroxidases belong to the second group, and we will try to investigate their activity practically.
Source of biological material
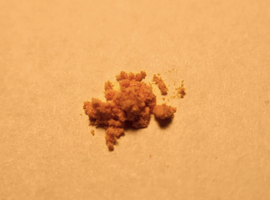
The best experimental source of peroxidase is horseradish root Armoracia rusticana from the cabbage family Brassicaceae (Fig. 1A). If horseradish is unavailable, common parsley root Petroselinum crispum, which belongs to the carrot family Apiaceae (Fig. 1B), can also be used.
Both plants are commonly used in cooking, so obtaining them should not be difficult. Acquiring horseradish root in the winter months is a bit more challenging, but it is possible to use parsley, which is available year-round (however, this may result in somewhat weaker results in the tests).
For all experiments, use fresh, unprocessed root tissue unless stated otherwise.
For Safety
While the joy of experimenting on your own is immense, it is important to consider potential hazards – especially since we will be using many substances that could be hazardous for various reasons. Benzidine and its derivatives (including o-tolidine) are highly toxic and carcinogenic. Luminol and phenolphthalein are also suspected to have similar properties. Sodium hydroxide, hydrogen peroxide, and acetic acid solutions with relatively high concentrations are corrosive and may cause permanent bodily harm upon direct contact. The latter also has an unpleasant choking odor and exhibits toxic effects when inhaled. In the case of finely powdered metallic zinc, the danger is of a different nature, as it may be highly flammable – in contact with certain oxidizers (e.g., ammonium nitrate(V)), it can even ignite spontaneously.
Another issue is safety when heating even small amounts of substances, particularly corrosive ones – this should be done carefully and gently, never directing the test tube’s opening towards people or animals.
I recommend reviewing the material safety data sheets (MSDS) for these substances to understand their hazards and properties.
For the reasons mentioned above, it is essential to conduct preparations, experiments, and clean-up in a responsible and careful manner. There is no room for improvisation here. Personal protective equipment is required: a lab coat, gloves, and safety glasses, and when heating corrosive substances, face protection is recommended.
Experiment I
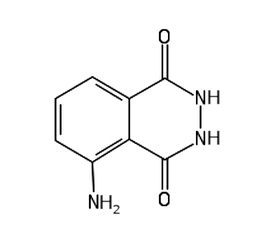
To test for the presence of peroxidase in root tissues, we will use luminol C8H7N3O2. It is an organic compound, the hydrazide of 3-amino-phthalic acid, with the structural formula shown in Fig. 1.
Luminol is typically a fine crystalline powder that ranges in color from yellowish to light brown (Fig. 2).
We need to prepare the appropriate solution by dissolving 1g of sodium hydroxide NaOH in 50 cm3 (1.69 fl oz) of distilled water and a small amount (on the order of milligrams) of luminol. This solution is not very stable, so it is best to prepare it fresh. To the resulting clear liquid, we add 1.5 cm3 (0.05 fl oz) of 30% hydrogen peroxide (perhydrol) or an appropriately larger amount of 3% pharmacy-grade hydrogen peroxide just before performing the test [1].
The horseradish or parsley root, after being washed and dried, should be grated. A small amount of material is enough (Fig. 3).
The plant material should be transferred into the solution (Fig. 4). At this point, you can observe the formation of small amounts of oxygen from the breakdown of hydrogen peroxide in contact with substances found in plant tissues.
To observe the reaction clearly, the room must be darkened. We will then observe that the solution, upon contact with the grated root, begins to glow very clearly. A simple stir of the solution will cause the entire contents of the vessel to emit bright, easily observable blue light (Fig. 5). The emission of bright light can last anywhere from several to a dozen seconds, while weaker light may last longer.
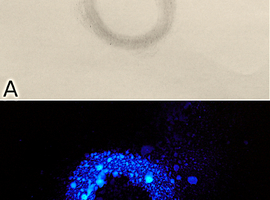
A variation of this experiment involves using a cut root or an aqueous tissue extract to draw patterns on paper (Fig. 6A). After spraying the previously prepared working solution, you can admire the glowing patterns on the paper (Fig. 6B). The result of the experiment will depend on how much peroxidase we managed to apply to the paper.
This experiment is visually striking and can be demonstrated to a large audience. As mentioned earlier, it is necessary to be able to darken the room.
Experiment II
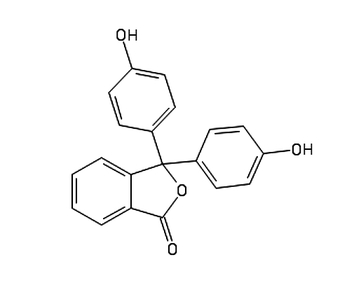
In this experiment, we will use phenolphthalein C20H14O4, a commonly used chemical in chemistry and biology labs (Fig. 2).
Phenolphthalein is a white or slightly yellowish powder (Fig. 7), poorly soluble in water, but much better in alcohols.
We know that phenolphthalein is used as an acid-base indicator because it shows no coloration in acidic and neutral environments, but in alkaline solutions, it turns pink or, at high concentrations, violet (Fig. 8).
To prepare the experiment, we need to make the appropriate solution by dissolving 0.1g of phenolphthalein in 10 cm3 (0.34 fl oz) of 25% sodium hydroxide solution [2].
The next required substance is powdered zinc Zn in the form of a dark gray, loose powder.
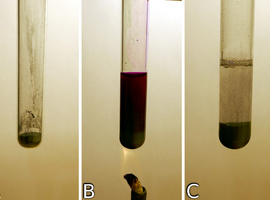
We place some zinc powder into a test tube, supported in a suitable holder (Fig. 9A). Then, the prepared phenolphthalein solution is added to the test tube, mixed with zinc, and gently heated using an electric heater or a spirit lamp (Fig. 9B). The purple liquid should gently boil. All steps must now be performed very carefully, as we are dealing with a boiling, highly caustic liquid. After a while, the liquid will be completely decolorized – at this point, stop heating and allow the residue of zinc and reaction products to settle (Fig. 9C).
The cooled, decolorized base solution can be stored for a long time in a tightly sealed brown glass container with a little fresh zinc powder at the bottom. The working solution is made by mixing one volume of the base solution (taken from above the zinc residue) with 9 volumes of 70% ethyl alcohol. This reagent is unstable and must be prepared just before use in the experiment.
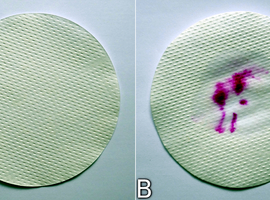
To detect peroxidase in the root, apply some plant extract to clean (essential, as some contaminants can interfere with the results) filter paper, such as a filter disk. Often, simply pressing the peeled root onto the paper will suffice. After drying, no trace of any substances should be observable on the paper (Fig. 10A).
If, however, the filter impregnated with peroxidase is wetted with the working solution, followed by hydrogen peroxide, you will notice the relatively quick appearance of the characteristic coloration for phenolphthalein in an alkaline environment (Fig. 10B).
In my experiment, I used freshly dug horseradish root – in other cases, the result may be subtler than the one shown in the photograph, and in some circumstances, it may even be difficult to detect (for example, if the root was stored for too long or at the wrong temperature). Similarly, if the root, even when fresh, contains less enzyme for some reason. In such cases, you can try directly wetting the root tissues with some water and check whether the described coloration appears.
The appearance of color after a longer time or its complete absence should be interpreted as a negative result. In some cases, despite the presence of peroxidase, the result may not confirm this for various reasons – one of them could be the presence of substances that interfere with the reaction.
Experiment III
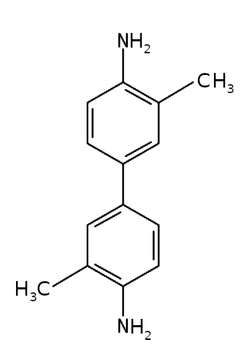
This time, the substance we will use is benzidine or some of its derivatives, such as salts. Dissolve 0.5-1g of this substance in 20 cm3 (0.68 fl oz) of heated concentrated acetic acid CH3COOH, then dilute it with distilled water to a volume of 50 cm3 (1.69 fl oz) and add 1 cm3 (0.034 fl oz) of perhydrol [3]. In my experiments, I used o-tolidine C14H16N2 (Fig. 3) instead of benzidine [4].
Next, two slices of parsley root were cut to about 5mm thickness (Fig. 11). One was left raw, while the other was blanched by immersing it in boiling water for about one minute, then cooled to room temperature.
Both slices should then be immersed in the solution (Fig. 12). You can also moisten the plant material placed on a Petri dish with the solution.
After a short while, the first signs of a chemical reaction can be observed: one of the slices starts to change color. Soon after, we can remove the slices from the solution, rinse them thoroughly under running water, and compare them. The differences are striking: the untreated slice has taken on a dark blue, almost navy color, while the blanched one shows no changes, and may even have become slightly decolorized (Fig. 13).
Thus, the observations reveal that after treatment with the prepared reagent, plant tissues containing peroxidase undergo coloring, but only if they have not been exposed to high temperatures.
Explanation
Peroxidases are a broad group of enzymes classified as oxidoreductases. They catalyze the oxidation of many different substrates using hydrogen peroxide. This reaction can be summarized as follows:
The cofactor in the described enzyme is heme, which has a porphyrin structure with an iron atom Fe at its center. Although hydrogen peroxide is the most commonly used substrate for peroxidases, some can also use other substrates, such as organic peroxides.
Enzymes from this group can be divided into three classes:
- I – bacterial peroxidases and ascorbate peroxidase,
- II – fungal peroxidases,
- III – peroxidases found in higher plant tissues [5].
In these experiments, we observed the activity of class III peroxidases. Horseradish peroxidase (HRP) is widely used for various commercial purposes.
Enzymes with similar actions also occur in animals. An example is glutathione peroxidase.
The luminol used in the first experiment belongs to a class of chemical substances that exhibit chemiluminescence, meaning the emission of light during chemical transformations. In this specific case, the oxidation of luminol by hydrogen peroxide produces a product initially existing in an excited, high-energy state. However, this situation is unstable, and the excited state is quickly reduced to the lower-energy ground state. According to the principle of energy conservation, the energy difference is then emitted into the environment in the form of light, here blue light. Importantly, in an aqueous environment, this reaction proceeds very slowly, and the emitted radiation is virtually undetectable. The reaction is accelerated by many catalysts, both inorganic, such as potassium ferricyanide K3[Fe(CN)6], and organic, such as those containing heme in their structure. It is no surprise, then, that peroxidase can oxidize luminol with hydrogen peroxide, as we observed in the experiment.
The situation is slightly different in the second experiment. The purple phenolphthalein (in an alkaline environment), when heated in the appropriate solution with metallic zinc, is reduced to colorless phenolphthalein, also known as leukophenolphthalein – we thus observe decolorization. Phenolphthalein, however, is susceptible to oxidation (which is why we store its solution in constant contact with reducing zinc). If there is an oxidizing catalyst, such as hydrogen peroxide, present in the sample, the colorless compound will be re-converted back into the purple phenolphthalein under these conditions, which we observed.
The described reaction with phenolphthalein – like the previous one with luminol – is commonly used to detect blood, or more precisely, the hemoglobin contained in erythrocytes, because the heme in its structure also catalyzes the described reactions. This is used, for example, in forensic science, and the reagent prepared in the manner described in the article is known as the Kastle-Meyer reagent.
It should be noted that according to some researchers, peroxidase itself may not reveal its presence during the phenolphthalein test [6]. In such cases, the observed effect would have to be due to another – probably also involving this enzyme, but more complex – oxidation mechanism.
Also, in the third experiment, the reason for the observed change was oxidation – this time, of the benzidine derivative into a colored product, in this case, blue. This happened only with tissue that had not been heat-treated. Peroxidase, as a holoenzyme, consists of a protein part (apoenzyme) and a non-protein part (heme as a cofactor). The three-dimensional structure is crucial for developing enzymatic activity, and high temperatures cause changes in the secondary, tertiary, and quaternary structures of the protein element. This obviously leads to the loss of biological activity. For this reason, the sample heated to the boiling point of water did not exhibit peroxidase activity.
The functions of peroxidases in organisms are very diverse, and it is not possible to list all of them here. However, it is worth mentioning that they seem to play a role, among other things, in the defense mechanisms that plants use against pathogens. In many members of the nightshade family Solanaceae, such as the eggplant Solanum melongena, it has been observed that the expression of genes responsible for the synthesis of guaiacol peroxidase begins within just a few minutes of bacterial infection [7].
Somewhat similar in action to peroxidase is catalase, described in the previous issue of Biologia w Szkole. However, catalase does not catalyze oxidation using hydrogen peroxide, but rather its decomposition [8].
References:
- [1] Ples M., Chemik na tropie - chemiluminescencja aktywowana krwią (eng. The Chemist on the Case – Chemiluminescence Activated by Blood), w serwisie: https://weirdscience.eu, online: http://weirdscience.eu/Chemik%20na%20tropie%20-%20chemiluminescencja%20aktywowana%20krwi%C4%85.html [25.01.2019] back
- [2] Ples M., Śledztwo – prosta metoda wykrywania obecności krwi (eng. Investigation – A Simple Method for Detecting Blood), Chemia w Szkole (Chemistry in School), 5 (2018), Agencja AS Józef Szewczyk, pp. 6-10 back
- [3] Pluciński T., Doświadczenia Chemiczne, Wydawnictwo Adamantan, 1997, pp. 92-93 back
- [4] Pluciński T., Peroksydaza, online: http://www.tomek.strony.ug.edu.pl/peroksydaza.htm [25.01.2019] back
- [5] Welinder K. G., Superfamily of plant, fungal and bacterial peroxidases, Current Opinion in Structural Biology, 2 (3), 1992, pp. 388-393 back
- [6] Ahmed S. A., Gowda H. S., Phenothiazines as reagents for the detection of faecal occult blood, Clinica Chimica Acta, 111(2-3), 1981, pp. 275-278 back
- [7] Prakasha A., Umesha S., Biochemical and Molecular Variations of Guaiacol Peroxidase and Total Phenols in Bacterial Wilt Pathogenesis of Solanum melongena, Biochemistry & Analytical Biochemistry, 2016, 5(292) back
- [8] Ples M., Katalaza - tlen trucizną (eng. Catalase - Oxygen as a Poison), Biologia w Szkole (Biology in School), 1 (2019), Forum Media Polska Sp. z o.o., pp. 60-63 back
All photographs and illustrations were created by the author.
Marek Ples