Proteins - The Building Blocks of Life
| Polish version is here |
The following article was originally published in the journal for educators Biologia w Szkole (Biology in School) (2/2017):

It is interesting that despite thousands of years of cultural development and countless efforts by philosophers and scientists, we still have not managed to formulate a definition of life that is indisputable, at least from our point of view.
The concept of life is most often defined in two, mutually complementary ways. First, it is defined as a set of so-called life processes, which are a highly organized collection of physical transformations and chemical reactions. These occur in systems called organisms, which are open from the thermodynamic standpoint, i.e., capable of exchanging both matter and energy with the environment. Organisms are hierarchically structured, composed of various numbers of cells (at least one), and engage in often highly complex biological processes [1]. Secondly, life is defined as a property specific to systems in which these processes take place [2].
When thinking about the definition of life, we usually encounter a significant problem quite quickly. Life is partly a process, not just a characteristic of complex physicochemical systems. Additionally, the definition of life should be broad enough to encompass all known living organisms, which are indeed very diverse [3].
Life can also be described as a property of a system (organism) that exhibits the following characteristics [4]:
- Homeostasis, meaning the ability to regulate the internal environment in such a way as to maintain the stability of its parameters.
- Metabolism, meaning the totality of chemical reactions and energy transformations that occur within the cells making up a living organism. Metabolic processes can be divided into anabolic (synthesis reactions) and catabolic (degradation reactions).
- Hierarchy, meaning the composition of one or more cells, which are the basic structural and functional units of living organisms.
- Growth, meaning the ability to maintain a higher level of anabolism than catabolism.
- Adaptation, meaning the organism's ability to adjust to new conditions over time.
- Responsiveness, meaning the ability to react to stimuli, both from the external and internal environments.
- Reproduction, meaning the ability to produce new generations of organisms.
Note that according to this definition, viruses, although exhibiting some characteristics of living organisms (e.g., the ability to replicate and adapt), are not considered living organisms because they lack a cellular structure and do not carry out their own metabolic processes.
Sometimes a reductionist definition of life is used, stating that life is a system or a collection of elements capable of evolution in the biological sense. However, some researchers criticize this definition for its excessive generality, as it would imply that certain computer programs are also alive. A good example here is the Tierra system [5].
All known living organisms have essentially the same chemical composition. The most important elements necessary for the existence of life on Earth are carbon (C), hydrogen (H), nitrogen (N), oxygen (O), phosphorus (P), and sulfur (S). Together, they form various organic compounds needed for life, including nucleic acids, sugars, and fats. A very important class of substances here are proteins, also called polypeptides.
In this article, I would like to introduce the reader to some issues related to the significance of proteins, their structure, and simple methods of detecting the presence of these substances in biological material.
Proteins – Extraordinary Substances
Proteins are macromolecular biopolymers or rather biopolycondensates made up of amino acid residues linked together by peptide bonds -CONH- (Fig. 1).
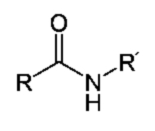
The peptide bond connects the α-amino group of one amino acid with the α-carboxyl group of another amino acid.
Amino acids, the building blocks of proteins, are a group of organic compounds containing a basic amino group and an acidic carboxyl group [6]. Natural proteins consist of 20 amino acids: alanine, arginine, asparagine, cysteine, phenylalanine, glycine, glutamine, histidine, isoleucine, aspartic acid, glutamic acid, leucine, lysine, methionine, proline, serine, threonine, tryptophan, tyrosine, and valine.
Typically, the number of amino acid residues in a single protein chain is greater than 100. Sometimes, proteins are made up of multiple chains called subunits.
Protein synthesis takes place in cells with the assistance of specialized structures – ribosomes [7].
The structure of a protein can be described at four hierarchical levels:
- Primary structure of a protein – the sequence of amino acids in a polypeptide chain.
- Secondary structure of a protein – the spatial arrangement of segments of polypeptide chains. Secondary structures include:
- alpha helix (ang. α-helix)
- beta sheet (ang. β-sheet)
- beta turn (ang. β-hairpin)
- Tertiary structure of a protein – the spatial arrangement of the secondary structure elements. It is determined by various chemical bonds and intermolecular interactions: disulfide bridges, ionic interactions, hydrophobic interactions, and van der Waals forces.
- Quaternary structure of a protein – the spatial arrangement and binding of protein subunits or prosthetic groups (in complex proteins).
Proteins serve many different functions. Some of them are structural substances, fulfilling a structural role. These include keratin, elastin, and collagen, which build specific structures, and cadherins, which are responsible for cell adhesion. Others, like actin and myosin in muscles, enable organized movement of the organism. For the transport of specific substances within the organism, hemoglobin and transferrin are involved, and for immune response, immunoglobulins. A very important area of protein activity is the regulation of biochemical processes in the organism, ranging from changes in the permeability of biological membranes to the regulation of growth and differentiation. Enzymes, which are biological catalysts, play an invaluable role here. They accelerate a wide variety of reactions, forming the chemical side of the phenomenon we call life [8].
Proteins are ubiquitous in the world of living matter. But how can we detect their presence and specific properties? Fortunately, there are many methods – in the next section, I will introduce a few of them. I have chosen methods that do not require special equipment or expensive reagents.
Detecting Proteins and Amino Acids
To begin the experiments, we need a convenient source of protein. Ovalbumin from eggs, such as chicken eggs, will work well. To prepare the experiment, dilute the chicken egg protein with distilled water about tenfold and filter it. The resulting solution is slightly yellow (Fig. 1) and should not be stored for too long.
Let's transfer a small amount of the protein solution to a test tube (Fig. 2). To visualize the expected changes, it is best to place the test tube against a dark background.
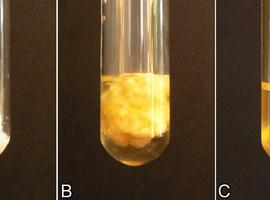
Next, add a few drops of ethanol (C2H5OH) with a concentration of at least 70% and stir. The effect is immediate – the protein solution becomes cloudy (Fig. 3).
If we decant or filter the precipitate and transfer it to distilled water, it will not dissolve again. The observed change is therefore irreversible.
The phenomenon observed is called protein denaturation. It involves changes in the second, third, and fourth protein structures under the influence of certain factors, while maintaining the amino acid sequence. The chemical and physical properties of the protein, including its solubility, change. Often, aggregation and precipitation processes are observed, as we have noted in this case.
Denaturing factors can be both physical and chemical. Physical factors include heating above a certain temperature (called the denaturation temperature), strong stirring, shaking, exposure to UV radiation, and high-intensity ultrasound. Chemical factors include the action of many alcohols (e.g., ethyl alcohol), heavy metal salts, strong acids and bases, guanidine chloride (CH6ClN3), and other substances.
The denaturing properties of ethyl alcohol have certain practical applications. It is, among other things, responsible for its antibacterial properties. Bacteria die as a result of protein denaturation in their cells, particularly in the cell membrane.
In addition to denaturation, there is a similar phenomenon called salting out proteins. In this case, proteins precipitate from the solution under the influence of solutions of light metal salts and ammonium. The key difference is that this process is reversible, i.e., after transferring the precipitate to a solution that does not contain the salting-out (or denaturing) agent, the protein will dissolve again. In this case, there is no damage to the protein chains, and the phenomenon is the result of disturbance of the solvation shell and protein aggregation.
Let's now look at characteristic reactions that can be applied to proteins. One of the most important is the biuret reaction.
The biuret reaction was first described by Ferdinand Rose in 1833 [9]. Just over 20 years later, it was independently described by the Polish physiologist Gustaw Piotrowski, and that is why it is also referred to as Piotrowski's reaction [10].
The biuret reaction allows for the detection of peptide bonds in various organic compounds, mainly proteins.
To prepare the reagent, we need:
- copper(II) sulfate pentahydrate CuSO4·5H2O
- sodium hydroxide NaOH
- potassium sodium tartrate (Seignette's salt) KNaC4H4O6
Aqueous solutions of sodium hydroxide NaOH are highly caustic. Avoid contact with the skin.
To prepare the reagent, dissolve 0.75g (0.026 oz) of blue crystals of hydrated copper(II) sulfate CuSO4·5H2O in 50cm3 (1.7 fl. oz) of distilled water, then add 10cm3 (0.34 fl. oz) of 50% aqueous sodium hydroxide NaOH. This produces a large amount of blue copper(II) hydroxide Cu(OH)2 precipitate. Next, add 2.5g (0.088 oz) of potassium sodium tartrate KNaC4H4O6 and mix thoroughly. The precipitate dissolves, forming a clear copper tartrate complex solution. Finally, dilute the solution with distilled water to 100cm3 (3.4 fl. oz). The resulting biuret reagent has a beautiful intense blue color (Fig. 4).
The reagent is stable and can be stored for a long time in a closed container.
The biuret reaction is named after biuret, a derivative of urea CO(NH2)2. Urea is the final product of the transformation of proteins and other nitrogenous compounds in ureotelic animals – it is excreted primarily with urine, in small amounts also with sweat. In its pure form, it is a white crystalline substance (Fig. 5).
When heated, urea undergoes a condensation reaction, resulting in a dimer, biuret H2NC(O)NHC(O)NH2 as shown in the reaction equation presented in Fig. 2.
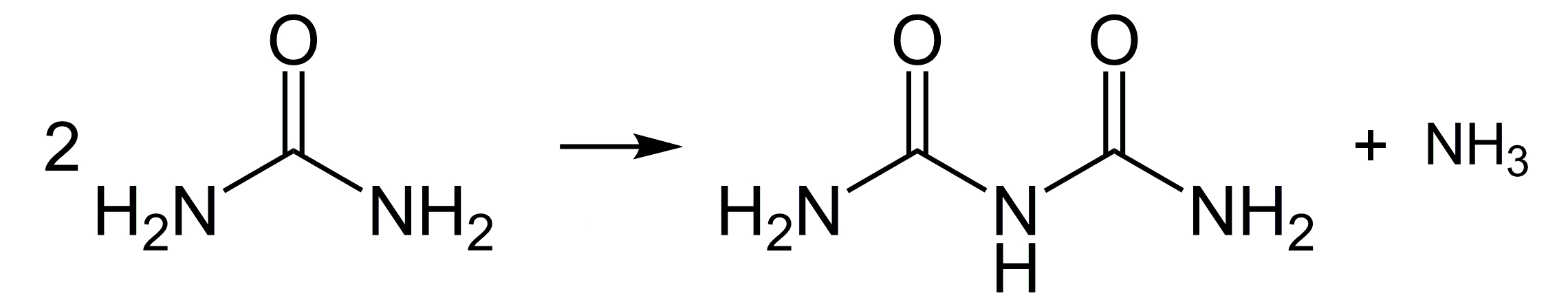
Biuret is not considered a protein, but due to its structure, it is the simplest chemical compound that undergoes the reaction named after it. We can easily verify this.
Place a few cubic centimeters of biuret reagent in two test tubes. In the first, add a pinch of urea, and in the second, add a similar amount of biuret (formed by heating urea). Stir both liquids until the substances dissolve.
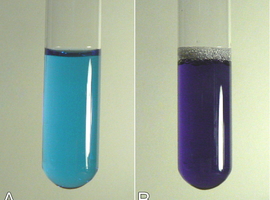
Almost immediately, an interesting observation can be made. The liquid in the test tube with added urea retains the natural blue color of the biuret reagent. In the second, a clear color change to violet can be observed, which we recognize as a positive result of the test (Fig. 6). As we can see, urea does not produce any effect in this case, unlike its dimer.
The biuret reagent, however, was intended for detecting proteins. We will use the previously prepared chicken egg protein solution. The control test will be the biuret reagent without added protein (Fig. 7A). In the second test tube, add just a few drops of the protein solution, and the reagent will turn a very intense violet color, much more intense than the previous result with biuret (Fig. 7B).
The color change is due to the formation of complex compounds in which the copper(II) ion Cu2+ is complexed by at least two peptide groups [11].
The biuret reaction can be used for qualitative and quantitative analysis. In the latter case, the intensity of the color of the solution is related to the concentration of the protein.
The biuret reaction is used, among other things, to check for the presence of free protein in bodily fluids, such as blood. The presence of large amounts of such protein may indicate serious internal organ damage.
Another characteristic reaction for proteins is the xanthoproteic reaction (from Greek ksanthós – yellow). We will try to use it to detect protein in cottage cheese (Fig. 8). Nitric acid (HNO3, Cp=65%) and ammonium hydroxide (NH3(aq)) are used. Remember that nitric acid is highly corrosive, and during the reaction, toxic nitrogen oxides may be released. Ammonium hydroxide releases ammonia gas (NH3), which is irritating and toxic in larger amounts. Caution is recommended!
Place a piece of cottage cheese in a test tube (Fig. 9A). Naturally, the color of the cottage cheese is white. Next, add a few cubic centimeters of nitric acid and gently heat. Almost immediately, the cottage cheese turns a light yellow color (Fig. 9B). After removing the acid (e.g., with a Pasteur pipette), moisten the sample with a diluted aqueous solution of ammonia. The result will be a deepening of the color, turning it orange (Fig. 9C). These color changes are characteristic for all protein substances [12].
The essence of the xanthoproteic reaction is the nitration of amino acid residues that contain aromatic rings (tyrosine, tryptophan, phenylalanine). Nitro derivatives are produced, which have a characteristic yellow color (Fig. 3).
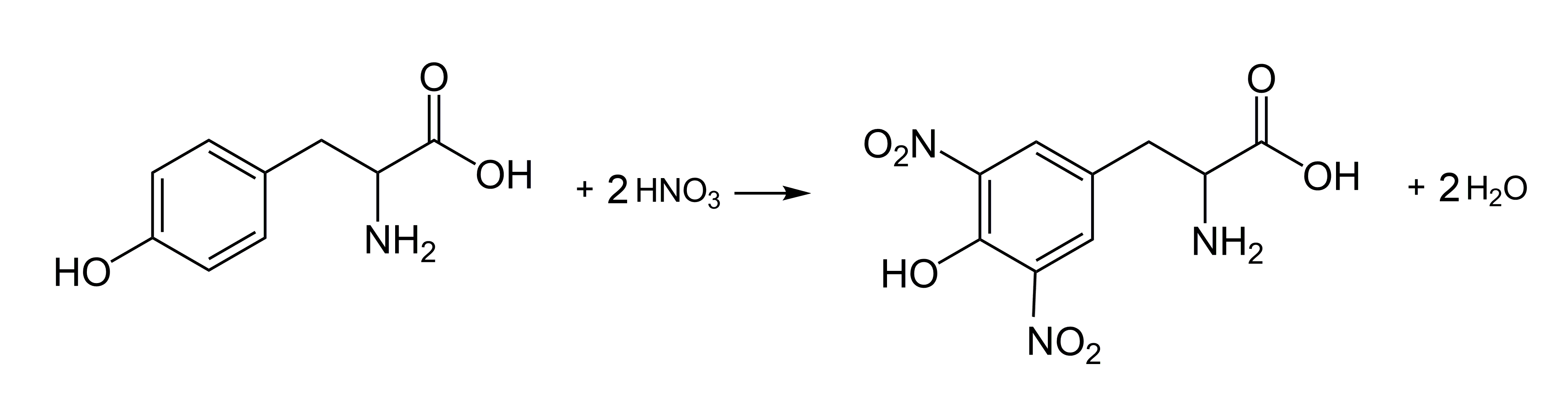
In an alkaline environment, such as under the influence of ammonia, the resulting nitro compounds convert into more intensely colored derivatives.
The next reaction I want to present is the so-called ninhydrin test. This is an extremely sensitive characteristic reaction that allows detection of free amino groups. It can be used to qualitatively and quantitatively determine the amino acids that build proteins.
Ninhydrin (C9H6O4) is an organic chemical compound. At room temperature, it is a white crystalline substance. It is harmful to health, so direct contact with it should be avoided. Ninhydrin is not very soluble in water and alcohols.
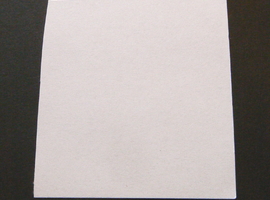
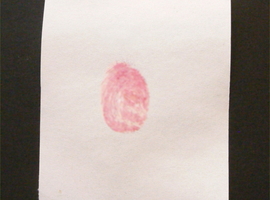
This compound is widely used to detect and quantify amino acids. Its high sensitivity has also made it useful in forensic science for revealing fingerprints. To verify this, let's take a piece of paper and make a fingerprint. The resulting fingerprint is, of course, invisible (Fig. 10).
Next, moisten the paper with a 0.1% solution of ninhydrin in alcohol and allow it to dry. At this stage, no changes are yet visible (Fig. 11).
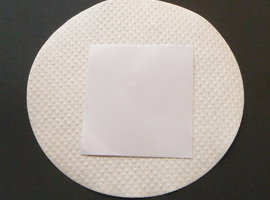
To reveal the fingerprint, gently heat the sample in the presence of steam. You can use a steam iron set to a low temperature. Place the paper between two layers of blotting paper. If using an iron without steam capabilities, gently moisten both blotting paper layers with water.
During this procedure, you will quickly notice the appearance of the fingerprint lines, forming the fingerprint image. The color of the developing image is red (Fig. 12).
The mechanism of this reaction is quite complex. Primary amines, such as amino acids, react with ninhydrin to form a Schiff base. This compound then breaks down. The resulting derivative reacts with another ninhydrin molecule, forming another Schiff base with an intense color, called Ruhemann's purple [13].
On the skin, for example, due to sweat secretion, there are always small amounts of proteins and amino acids. Their amount is sufficient for us to detect them using the described method.
Always wear gloves when working with ninhydrin solutions. This is not only due to its toxicity but also because it leaves hard-to-remove stains on the skin.
Summary
It is no exaggeration to say that proteins play a fundamental role in all biological processes. They serve a structural function. As enzymes, they participate in catalyzing transformations in biological systems – thus shaping the entire metabolism. We must also remember that they are involved in the transport of various particles, serve as antibodies, and participate in the transmission of nerve impulses.
Through the described simple experiments, it is possible to introduce students to methods of detecting proteins and the properties of these substances. I believe this is a valuable educational opportunity.
References:
- [1] Gánti T., Fundamentals of Life, Wiedza Powszechna, Warsaw, 1986, pp. 76, 80 – 81 back
- [2] Chmurzyński J. A., In Search of the Essence of Life, in: Organism - Biological Unit, Państwowe Zakłady Wydawnictw Szkolnych, 1977, pp. 65 back
- [3] Nealson K. H., Conrad P. G., Life: Past, Present and Future, Philosophical Transactions of the Royal Society B, 354 (1392), 1999, pp. 1923 - 1929 back
- [4] Habitability and Biology: What are the Properties of Life?, in: http://phoenix.lpl.arizona.edu/, archived online: https://web.archive.org/web/20220514054254/https://www.lpl.arizona.edu/missions/phoenix [accessed 28.01.2017] back
- [5] Bedau M. A., McCaskill J. S., et al., Open Problems in Artificial Life, Artificial Life, 6 (4), 2000, pp. 363 - 376 back
- [6] Mastalerz P., Organic Chemistry, Wydawnictwo Naukowe PWN, Warsaw, 1986, pp. 966 back
- [7] Berg J. M., Tymoczko J. L., Stryer L., Clarke N. D., Biochemistry, Wydawnictwo Naukowe PWN, Warsaw, 2007 back
- [8] Ples M., Enzymy - biologiczne katalizatory (eng. Enzymes: Catalysts of Life), Chemia w Szkole (eng. Chemistry in School), 3, 2016, Agencja AS Józef Szewczyk, pp. 6 - 11 back
- [9] Rose F., On the Compounds of Protein with Metal Oxides, Annalen der Physik und Chemie, 104 (5), 1833, pp. 132 - 142 back
- [10] von Piotrowski G., A New Reaction for Protein Bodies and Their Derivatives, Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften, Mathematisch-Naturwissenschaftliche Classe, 24, 1857, pp. 335 - 337 back
- [11] Freeman H. C., Smith J. E. W. L., Taylor J. C., Crystallographic Studies of the Biuret Reaction. I. Potassium Bis-Biuret Cuprate(II) Tetrahydrate, K2[Cu(NHCONHCONH)2]·4H2O, Acta Crystallographica, 14, 1961, pp. 407 - 418 back
- [12] Sękowski S., In the Circle of Nitrogen (from the series: Chemistry in Daily Life), Młody Technik, 11 (208), 1965, pp. 112 - 115 back
- [13] Wigfield D. C., Buchanan G. W., Croteau S. M., On Ruhemann's Purple, Canadian Journal of Chemistry, 58 (3), 1980, pp. 201 - 205 back
All photographs and illustrations were created by the author.
Marek Ples