Preparation of Iron Oxalate
| Polish version is here |
Iron oxalate is a salt formed from oxalic acid and iron. Like most oxalate compounds, it is relatively insoluble in water. Iron oxalate is often used in a fascinating experiment involving spontaneously igniting iron powder (described here). You can buy this reagent from a chemical supply store or synthesize it yourself easily. Naturally, today we're choosing the DIY approach!
Required Substances
To prepare iron oxalate, you only need two substances:
- Oxalic acid dihydrate, C2H2O4•2H2O
- Iron(II) sulfate heptahydrate, FeSO4•7H2O
Warning: As always, exercise caution when working with chemicals. The reagents used in this experiment may be irritating or harmful. The author assumes no responsibility for any injuries or damage resulting from performing this experiment. Proceed at your own risk!
Oxalic acid, the simplest dicarboxylic acid, appears as a crystalline powder in its dihydrate form. Iron(II) sulfate heptahydrate forms greenish crystals.
The Synthesis!
Once you have these two substances, you can start synthesizing iron oxalate. First, prepare concentrated (or relatively strong) solutions of oxalic acid and iron(II) sulfate. Next, mix these solutions together. Heating the mixture significantly accelerates the reaction; the solution quickly becomes cloudy, and a yellow iron oxalate precipitate begins to settle at the bottom. You can watch the reaction in this video:
Then, wash the precipitate several times by decantation, filter it, and dry it thoroughly. Drying should be carried out at room temperature (around 20 °C [68 °F]) because higher temperatures may cause decomposition. The finished iron oxalate appears as a light-yellow powder:
Explanation
Chemically, this is a simple double-displacement reaction. The net ionic equation is shown below:
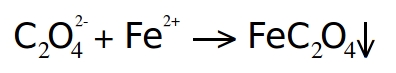
The resulting iron oxalate is practically insoluble in water and precipitates as a yellow solid.
Have fun, and enjoy learning something new!
Further readings:
- Bielański A., Chemia ogólna i nieorganiczna, PWN, Warszawa, 1981
- Trzebiatowski W., Chemia nieorganiczna, Wyd. VIII, PWN, Warszawa, 1978
- Vogel A. I., Tatchell A.R., Furnis B.S., Hannaford A.J., Smith P. W. G., Vogel's Textbook of Practical Organic Chemistry, 5th Edition, Prentice Hall, 1996
Marek Ples