Who Mixed the Sugars? A Brief Analysis
| Polish version is here |
The following article was originally published in the journal for educators Biologia w Szkole (eng. Biology in School) (1/2018):

Analysis
The concept of the life force, or vis vitalis in Latin, stems from alchemical ideas and was introduced in the 16th century by Jan Baptist van Helmont, a Flemish physician and physiologist. Many believe it was he who initiated the process that ultimately transformed alchemy into chemistry as we know it today [1]. The term vis vitalis referred to an unspecified mysterious force present in living organisms. It was thought to grant them the ability to synthesize organic chemical compounds.
The notion of a life force arose because, although the existence of specific chemical compounds in living organisms had been confirmed, researchers initially could not synthesize them under laboratory conditions. It was not until 1828 that Friedrich Wöhler successfully produced the organic compound urea CO(NH2)2 from inorganic substrates such as ammonia NH3 and cyanic acid HOCN [2]. In doing so, he demonstrated that vis vitalis was not essential for the formation of these compounds. Consequently, this date is often considered the starting point of modern organic chemistry.
Finding a single method to classify the vast number of organic compounds poses many challenges. One need only note that defining them simply as chemical compounds containing carbon leads to numerous interpretative problems. Simple substances (carbon dioxide CO2, carbonic acid H2CO3, hydrogen cyanide HCN, and others) are not classified as organic compounds. Other, more complex definitions can also be somewhat imprecise. This is due to the fact that no strict boundary exists between organic and inorganic compounds.
Beyond any doubt, however, carbohydrates — often referred to as sugars or saccharides — are classified as organic compounds. They consist of carbon C, hydrogen H, and oxygen O, typically in a ratio of H:O = 2:1. Their structure commonly includes hydroxyl, carbonyl, and hemiacetal groups.
Carbohydrates can be divided, among other ways, into:
- monosaccharides,
- oligosaccharides,
- polysaccharides.
Monosaccharides can also be distinguished by the number of carbon atoms they contain. For instance, there are trioses (3 carbons, e.g., glyceraldehyde C3H6O3), tetroses (e.g., threose C4H8O4), pentoses (e.g., ribulose C5H10O5), hexoses (e.g., glucose C6H12O6), and heptoses (e.g., mannoheptulose C7H14O7).
Another way to classify simple sugars is by their functional groups:
- ketoses – containing a ketone group (=C=O),
- aldoses – containing an aldehyde group (-CHO).
Ketoses include, for example, the aforementioned ribulose, while aldoses include glucose.
Oligosaccharides and polysaccharides form when two or more monosaccharides are joined by a glycosidic bond. In this way, glucose and fructose combine to produce sucrose C12H22O11, which is widely used as table sugar.
Most sugars we encounter in daily life look quite similar — at room temperature they are typically white, crystalline or amorphous solids. Despite their similar appearance, sugars often differ significantly in their chemical properties. For that reason, I would like to describe simple methods of distinguishing sugars that can be used successfully in a school or university lab. Such a basic chemical analysis of biologically active compounds can be an excellent way to illustrate the connection between two natural sciences: biology and chemistry.
Identification
In this experiment, we will use three readily available sugars: sucrose, glucose, and fructose. When ground together in a mortar to form a uniform powder, these three sugars are practically indistinguishable to the naked eye. As shown in Photo.1, one cannot tell them apart by looks alone. I will not yet reveal which of the sugars (Photo.1A, B, C) is sucrose, glucose, or fructose.
All the sugars shown have a sweet taste and dissolve well in water. To simplify interpretation in all trials and their accompanying photos, we will keep the same labels as in Photo.1.
To distinguish these substances, chemical methods must be employed. We will use characteristic reactions, beginning with Fehling’s test, which detects reducing compounds. It was developed in the 19th century by the German chemist Hermann von Fehling [3].
The use of Fehling’s test to distinguish sugars relies on the fact that some sugars can reduce other substances. Of the sugars we will use, both glucose and fructose are reducing sugars — unlike sucrose.
The main ingredient needed for this test is a copper(II) salt, specifically copper(II) sulfate(VI) pentahydrate CuSO4 • 5H2O. It is a crystalline solid with a striking blue color (Photo.2).
Interestingly, this substance was known to alchemists and called copper vitriol (Lat. Vitriol coeruleum). This name may also evoke the Latin motto: Visita Interiora Terrae Rectificando Invenies Occultum Lapidem (Visit the interior of the earth and purifying you will find the hidden stone).
To perform Fehling’s test, two solutions must first be prepared. Their composition is as follows:
- Solution A – 2 g (0.07 oz) of NaOH and 1 g (0.035 oz) of NaKC4H4O6 in 50 cm3 (1.69 US fl oz) of H2O
- Solution B – 2 g (0.07 oz) of CuSO4 • 5H2O in 40 cm3 (1.35 US fl oz) of 3% H2SO4
Of course, distilled water should be used to prepare these solutions.
While the sodium potassium tartrate NaKC4H4O6 (Rochelle salt) is not toxic, you should exercise caution when handling the other substances. Copper(II) sulfate(VI) may be harmful, and both sulfuric(VI) acid and sodium hydroxide are corrosive — they can cause severe burns and, if they come into contact with the eyes, can lead to permanent damage.
Solution A is colorless, whereas Solution B has a blue color thanks to the presence of copper ions (Photo.3).
Both solutions can be stored for a long time in sealed containers. Small bottles made of glass or plastic, which allow drop-by-drop dispensing, are very convenient (Photo.4). Of course, they must be properly labeled.
Fehling’s reagent is prepared immediately before the test by mixing Solutions A and B in appropriate proportions.
In a test tube, place a few cubic centimeters of Solution A and, while stirring, add a few drops of Solution B. Fehling’s reagent is ready when the solution acquires a stable blue color (somewhat more intense than the color of Solution B alone).
We want to identify three sugars. Thus, we take the same number of test tubes, each freshly prepared with Fehling’s reagent. To each one, add a pinch of the sugar sample. After the solids dissolve, no visible difference can be observed (Photo.5).
To observe the characteristic differences, the test tubes must be heated in a water bath or with a spirit burner (Photo.6).
If using a burner, be careful to avoid splattering the hot liquid.
After just a short time, an interesting phenomenon occurs — the solution changes from blue to an orange-red color and becomes turbid. However, this color change appears only in the first and second test tubes. In the third tube, the solution remains blue and clear despite heating (Photo.7).
The absence of a color change (negative result) in sample C clearly indicates that it contained sucrose. In this way, we have identified that sugar.
Fehling’s test, however, does not distinguish glucose from fructose, as both give a positive result. Another test must be used for that purpose.
To easily identify these sugars, we can take advantage of the fact that glucose is an aldose while fructose is a ketose. Differences in their chemical properties can be demonstrated using the so-called Seliwanoff’s test, developed by the Russian chemist Fedor Seliwanoff in 1887 [4].
Preparing Seliwanoff’s reagent is not difficult. It requires hydrochloric acid HCl with a concentration of about 18%. Another necessary substance is 1,3-dihydroxybenzene C6H4(OH)2, whose structural formula is shown in Fig.1.
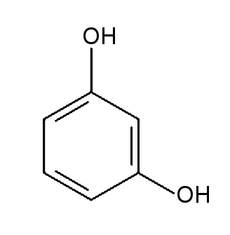
This substance is often called resorcinol. Under normal conditions, it appears as a white powder or flakes that gradually develop a subtle pinkish tint when exposed to air (Photo.8).
To prepare Seliwanoff’s reagent, add a few drops of an alcoholic solution of resorcinol to a small amount of suitably concentrated hydrochloric acid. The resulting solution is essentially colorless (Photo.9).
We will place this solution into test tubes and then add a pinch of each substance being tested. As with Fehling’s reaction, at room temperature no differences in appearance can be noted (Photo.10).
Again, heating the samples is necessary. A simple water bath, for instance using a larger beaker of hot water (Photo.11), will suffice.
Within just a few seconds, you can observe that one of the test tubes quickly takes on a deep red color. The result of the test is shown in Photo.12.
A positive result is interpreted as the rapid development of an intense color (Photo.12B). A slight color change (Photo.12A) after prolonged heating — or its complete absence — should be interpreted as negative.
A positive result indicates that the tested sugar was a ketose, meaning sample B contained fructose.
Combining the information gained from both Fehling’s and Seliwanoff’s tests, we can conclude that labels A, B, and C (vide Photo.1) corresponded, respectively, to glucose, fructose, and sucrose.
As a sort of complement, I would like to include the structural formulas of these sugars, now that we know their identities (Fig.2).
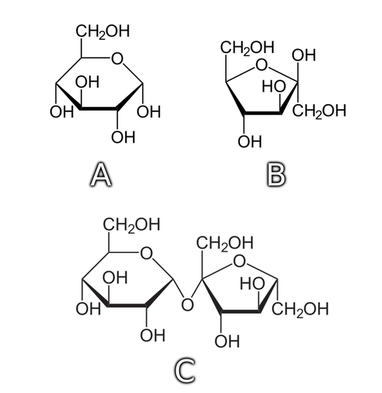
Explanation
In Fehling’s reaction, copper(II) ions Cu2+ — shown as free ions but actually present in tartrate complexes — react under alkaline conditions with a compound containing an aldehyde group. Thus, the metal is reduced, forming insoluble copper(I) oxide Cu2O with a red-orange color:
Meanwhile, the aldehyde is oxidized to the corresponding carboxylic acid, or more precisely (due to the presence of a base), its sodium salt [5]. In the case of sugars, this produces sodium salts of aldonic acids (e.g., sodium gluconate).
You might think this method should only detect the reducing properties of aldoses — and thus also differentiate them from ketoses, which lack an aldehyde group. Unfortunately, it does not, because many ketoses undergo keto-enol tautomerization and can exhibit reducing properties similar to those of aldoses.
As we see, most simple sugars and disaccharides give a positive Fehling’s test. An exception is sucrose, which we used to our advantage in the experiment. Polysaccharides generally yield a negative result because of the low number of reducing ends in their long polymer chains.
Seliwanoff’s test, on the other hand, relies on the fact that ketoses transform under hydrochloric acid much more readily into furfural derivatives than aldoses do. The derivative formed then reacts with resorcinol to produce a red product. Aldoses can undergo a similar reaction but do so far more slowly. Thus, the method can distinguish ketoses from aldoses [6].
It should be noted that sucrose, as a disaccharide composed of glucose and fructose residues, yields a positive Seliwanoff’s test.
References:
- [1] Encyclopedia Britannica, online: https://www.britannica.com/biography/Jan-Baptista-van-Helmont (15.12.2017) back
- [2] Wöhler F., Ueber künstliche Bildung des Harnstoffs, Annalen der Physik und Chemie, 88 (2), 1828, pp. 253-256 back
- [3] von Fehling H., Die quantitative Bestimmung von Zucker und Stärkmehl mittelst Kupfervitriol, Justus Liebigs Annalen der Chemie, 72, 1849, pp. 106-113 back
- [4] Seliwanoff Th., Notiz über eine Fruchtzuckerreaction, Berichte der deutschen chemischen Gesellschaft, 20, 1887, pp. 181 back
- [5] Fieser L. F., Fieser M., Advanced Organic Chemistry, Chapman & Hall, 1962, pp. 425 back
- [6] Katoch R., Analytical Techniques in Biochemistry and Molecular Biology, Springer-Verlag New York, 2011 back
All photographs and illustrations were created by the author.
Marek Ples











